
DR. VARUN KUSHWAH
Senior Research Scientist
Advanced Products and Drug Delivery
Fulbright and Commonwealth Fellow
My research aims to understand the concepts and mechanisms which underly the complexities of pharmaceutical science. My aim is to achieve the highest level of research attainment making advance medication possible to needy ones.

MY RESEARCH JOURNEY
Continuous learner
I am currently working as Senior Research Scientist at Research Center Pharmaceutical Engineering (Research industry affiliated with Technical University), Graz, Austria, where I am working in the dynamic and interdisciplinary projects encompassing pharmaceutical process optimization, pioneer advanced formulation product (early stage to late stage development), polymer assisted development in drug delivery and biomedical science, solid-state stability of chemicals/pharmaceutical products and BioMedical/Pharmaceutical device development. Before joining RCPE, I also had the opportunity to work in the prestigious pharmaceutical company (Pfizer) as a Research Scientist, where I worked in the resourceful domain of pharmaceutical parental product development.
During my academic journey, after completing B. Pharm from Dr. Hari Singh Gour Central University, I joined Department of Pharmaceutical Engineering and Technology, IIT BHU to purse M. Pharm (Pharmaceutics). I passed M. Pharm with Honors and cleared all the multilevel entrance examinations including GPAT, GATE and CSIR-NET. Thereafter, I pursued my PhD from Department of Pharmaceutics, National Institute of Pharmaceutical Education and Research (NIPER), Mohali. In-addition, I have also conducted a part of my Ph.D. research work in University of Louisville, Kentucky, USA and University of Strathclyde, Glasgow, Scotland, UK with the support of Fulbright-Nehru and Commonwealth split site Ph.D. program, respectively.
In PhD, I worked in the multifaceted domain of formulation, biomedical and chemical science and was actively engaged in the design, synthesis and biological evaluation of functional protein and polymer based nanomaterials for bio-medical applications and gained profound knowledge regarding preparation and characterization of numerous Nano particulate systems. In order to expand the frontiers of knowledge, during PhD, I also worked in several research/ academic collaborations with international and national universities.
After academics, in Industry (Pfizer) I value-added my interpersonal and organizational abilities and collaborated effectively with Multinational Companies and Universities globally in different research horizons of advanced innovative as well as generic products, process and BioMedical/Pharmaceutical instrument development.
Owing to collaborative research in partnership with science institutes and industries, as a part of team, I was able to bridge together the fundamental, applied sciences, material engineering, chemical engineering, and medicine to meet the challenge of developing cutting-edge research or technology worth millions of capital.

PUBLISHED WORK
Recent 5 publications
I’ve been fortunate to have worked on several different research projects in an array of interesting subjects, and I’m happy to say that my hard work paid off, and I have many scientific publications to show for it. Check out my list of published works below and contact me with any questions or for more information about my research.
27 April 2020
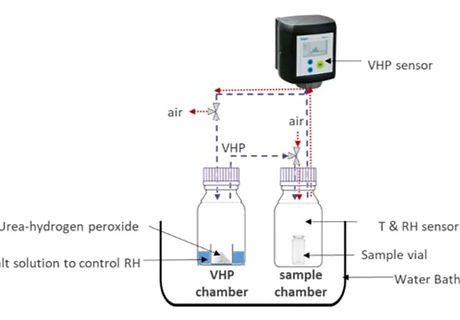
Isolators for aseptic filling of biopharmaceuticals and vaccine products are commonly sanitized by vaporized hydrogen peroxide (VHP). However, remaining traces of H2O2 may contaminate the solution and cause oxidative degradation of the pharmaceutical products. The present report aims to establish a thorough understanding of the factors influencing H2O2 adsorption on empty glass intended for pharmaceutical product filling. A lab-scale miniaturized set-up that mimics the VHP- based isolator decontamination process was used. A fractional factorial design of experiment (DoE) was performed including relative humidity (RH), VHP concentration and exposure time as variables. The results revealed that VHP concentration and RH both impacts significantly the extent of H2O2 adsorption on the surface of glass vials and rubber stoppers. The lower extent of H2O2 adsorption at elevated RH implies the existence of competitive co-adsorption. Thus, adsorbed H2O2 may be removed more efficiently from the isolator after the decontamination phase by insufflating air with a high %RH rate during the isolator's aeration phase. The understanding gained from the present set-up can be applied to optimize the design of isolator decontamination cycles and evaluate the trade-off between process performance and the resulting product quality.
30 April 2020
The present study investigates the drug release-governing microstructural properties of melt spray congealed microspheres encapsulating the drug crystals in the matrix of glyceryl behenate and poloxamer (pore former). The solid-state, morphology, and micromeritics of the microspheres were characterized, before and after annealing, using calorimetry, X-ray scattering, porosimetry, scanning electron microscopy, and, NMR diffusometry. The in vitro drug release from and water uptake by the microspheres were obtained. The extent and the rate of drug release from the microspheres increased with a high poloxamer content and at higher annealing temperature and RH. All the drug release profiles were describable using the Higuchi release kinetics pointing towards the diffusion controlled release, both before and after annealing. The annealing process led to the polymorphic conversion of lipid and the increase in the pore size, predominantly at a higher temperature and humidity and for a high poloxamer content. The poloxamer domain increased from an initial 300 nm, up to 2000 nm upon annealing. The water diffusion rate inside the annealed microsphere was twice as fast as for unannealed counterparts. The findings relate the overall phase and pore structure change of the microsphere to the increased drug release induced by annealing. This work serves as a basis for the rational understanding of the modification of the in vitro performance by annealing, a widely used post-process for solid lipid products.
26 December 2019
Mycophenolic acid (MPA) has promising anticancer properties; however, it has limited clinical applications in vivo due to hydrophobic nature, high first-pass metabolism, lack of targeting, etc. These associated problems could be addressed by developing a suitable delivery vehicle, inhibiting the first-pass metabolism and additive/synergistic pharmacodynamic effect. Thus, MPA loaded highly stable lipid polymer hybrid nanoparticles (LPNs) were developed and investigated with the combination of quercetin (QC), a CYP 450 inhibitor cum anticancer. LPNs of MPA and QC (size; 136 ± 12 and 176 ± 35 nm, respectively) demonstrated higher cellular uptake and cytotoxicity of combination therapy (MPA-LPN + QC-LPN) compared to individual congeners in MCF-7 cells. In vivo pharmacokinetics demonstrated 2.17 fold higher T1/2 value and significantly higher pharmacodynamic activity in case of combination therapy compared to free MPA. In nutshell, the combinatory therapeutic regimen of MPA and QC could be a promising approach in improved breast cancer management.
30 January 2020
The present work demonstrates the utility of temperature controlled set up with pressurized headspace oxygen as an approach to effectively reduce the time required for solid-state drug-excipient compatibility study. To illustrate the utility, the incompatibility of polyethylene glycol (PEG) and polyethylene oxide (PEO) with Famotidine (Fam) was shown. Owing to thermal and oxidative stress, polyethylene ether moieties of PEG generated reactive impurities, resulting in the degradation of Fam. The chemical degradation was evaluated via liquid chromatography. Around 20% of degradation was observed in the pressurized oxygen set up, whereas, no degradation was found in the absence of oxidative stress. On increasing the excipient fraction, the Fam degradation increased proportionally. Formation of aldehydes and free radicals from excipients were proposed as the precursors for Fam degradation. The generation of aldehydes and free radicals was confirmed by infrared and Electron Spin Resonance (ESR) spectroscopic analysis, respectively. Overall, the present study demonstrated the utility of pressurized oxygen set up as a rapid and routine tool for studying drug-excipient incompatibility at temperatures relevant drug-product manufacture.
12 October 2019
A modified facile biomimetic Temozolomide Chitosan nanogel (TCNL) was developed offering pH responsive, charge attracted and microenvironment dependent tumor targeting nanotherapy. USFDA approved chemotherapeutic TMZ (Temozolomide) was encapsulated in a cationic biocompatible chitosan nanogel subsequently surface modified with nonionic Transcutol by inotropic gelation method and evaluated for its combined anti-metastatic and antitumor efficiency. The in-vitro results authenticated that TMZ encapsulated TCNL was effectively uptake and distributed in HaCaT cell line inducing high apoptosis and necrosis of tumor cells prior to the electron microscopic (TEM & SEM) and thermal evaluations (DSC, DTA & TG) suggesting spherical and thermo-stable nanogel system. An accelerated sustained release pattern of TMZ from TCNL was displayed in mildly acidic conditions (pH 6) signifying ultra-sensitivity of TCNL. In-vivo evaluation over 16 week DMBA/croton oil tumor induced mice model showed noteworthy tumor targeting with down regulation of overexpressed COX-2, cytokines and nuclear factors on western blot analysis. Moreover, advanced gamma scintigraphy analysis displayed significant drug accommodation and expressing potent tumor accumulation, suppression and metastasis effect on carcinogenic mice. The TCNL outcomes displayed effective tumor targeting on transdermal delivery for operative nanotherapy against skin cancer.

MY RESEARCH
I am interested in studying the natural systems which shape and guide the processes of the natural world. My long-term goal is to identify and characterize the scientific mechanisms specific to my principal areas of research; Advanced drug delivery systems (nanotechnology, biodegradable/non-biodegradable implants, hot-melt extrusions, lipid-based microspheres, amorphous solid dispersion, osmotic-controlled release oral delivery system (OROS), 2D/3D printed formulation), Conventional (oral and parenteral including prefilled syringes) drug delivery system, Pharmaceutical stability science, BioMedical/Pharmaceutical instrumentation design, and development, Pharmaceutical reverse engineering, In-vitro cell culture/In-vivo animal model development and regenerative medicine.
Read more about these projects below.
ADVANCED DRUG DELIVERY SYSTEMS
CONVENTIONAL DRUG DELIVERY SYSTEM

PHARMACEUTICAL STABILITY SCIENCE
BIOMEDICAL/PHARMACEUTICAL INSTRUMENTATION DESIGN & DEVELOPMENT

PHARMACEUTICAL REVERSE ENGINEERING
IN-VITRO CELL CULTURE/IN-VIVO ANIMAL MODEL DEVELOPMENT

Showcase of Work

Effect of annealing on lipid microstruture & drug release

PLGA BASED IMPLANT
Intravitreal delivery

Efficient nuclear co-localization

Co-delivery of docetaxel and gemcitabine

Gemcitabine-docetaxel combinatorial dual drug conjugate

Gemcitabine Conjugated Albumin Nanoparticles
AWARDS AND RECOGNITION
FULBRIGHT-NEHRU DOCTORAL RESEARCH FELLOWSHIP
2016-2017 Fulbright-Nehru Doctoral Research Fellowship by United States-India Education Foundation (USIEF)
COMMONWEALTH SPLIT-SITE (PHD) SCHOLARSHIP
2016 Commonwealth Split-Site Scholarship by Commonwealth Scholarship Commission (CSC) in the UK
SCI SCOTLAND GROUP PHD STUDENT COMPETITION AWARD 2016
From Society of Chemical Industry, Scotland
COVER PAGE ARTICLE
International Journal Pharmaceutical research (volume:34, issue: 11, November 2017)
CSIR NET
All India Rank-043
GPAT
2011 & 2012
GATE
2012 & 2013
NIPER JEE
2011 & 2013
CONTINUING EDUCATION PROGRAMS/TEACHING
Item Subtitle





